Identification of the preparation method of the alleged infringing drug in patent infringement dispute
Identification of the preparation method of the alleged infringing drug in patent infringement dispute
——Eli Lilly & Co. Vs. Watson Pharmaceuticals (Changzhou) Co., Ltd[1]: Case of dispute over invention patent right infringement
[Judgment Highlight]
In the patent infringement dispute over drug preparation method, in the absence of other evidence to the contrary, the preparatory method of the alleged infringing drug filed with the pharmaceutical supervisory department should be presumed as its actual preparation method. In case there is evidence proving that the preparatory method of the alleged infringing drug that has been filed is not true, then evidence including the technical sources, production procedures, batch manufacturing records and filing documents of the alleged infringing drug should be fully reviewed in order to determine the actual preparation method of the alleged infringing drug in accordance with the law.
With respect to complicated technical facts such as the preparation method of the alleged infringing drugs, they can be verified by comprehensive application of various approaches, such as the use of technical investigators, expert advisors, judicial appraisal and technology expert consultation.
[Case Number]
Supreme People's Court (2015) MSZZ No.1
[Cause of Action]
Dispute over invention patent right infringement
[Keywords]
Invention patent right infringement, invention patent of drug preparation method, scope of protection, technical investigator, ascertainment of preparation methodology of alleged infringing drug
[Relevant Laws]
Paragraph 1, Article 56, Paragraph 2, Article 57 and Paragraph 1, Article 62 of Patent Law of the People’s Republic of China (2000 amendment), Articles 78 and 79 of Civil Procedure Law of the People’s Republic of China
[Basic Facts]
On July 25th, 2013, Lilly (also known as Eli Lilly and Company) filed a lawsuit to the Jiangsu High People’s Court (hereinafter referred to as Jiangsu High Court), claiming that Lilly has the invention patent right No. 91103346.7 for the preparation method of the case involved, and that the drug olanzapine prepared with the patent method involved, is a new product. Watson Pharmaceuticals (Changzhou) Co., Ltd (hereinafter referred to as Watson) produced olanzapine using the preparation method within the scope of patent right protection and sold it in the market, which has infringed upon the invention patent right for the involved method of Lilly. To this end, Lilly filed this lawsuit and requested the court order that: 1. Watson shall compensate Lilly for its economic losses of RMB151,060,000 and pay another RMB28,800 for the investigation fee and other reasonable expenses incurred by Lilly to deter the infringement. 2. Watson shall post a statement on its website and Medical Economics to eliminate the adverse effects of its infringement on Lilly. 3. Watson shall bear the attorney fee of RMB1,500,000 incurred by Lilly in this case. 4. Watson shall bear for all litigation costs incurred in this case.
The Jiangsu High Court, the court of first instance found that: the patent involved is the Chinese invention patent application No. 91103346.7, titled “Method to prepare a thieno- benzodiazepines compound”, which was applied by Lilly Industrial Company of the UK on April 24th, 1991. The patent right involved was authorized and proclaimed on February 19th, 1995 and expired on April 24th, 2011. The patentee of the patent involved was changed to Eli Lilly Ltd of the UK on March 17th, 1998 and then changed to Eli Lilly and Company on February 28th, 2002.
The claim of the authorized patent involved is: 1. A method to prepare 2-methyl-10-(4-methyl-1- piperazine)-4H-thieno-[2,3,-b][1,5]benzodiazepine, or add its acid into salt.
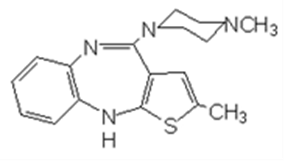
The described method includes:
(a) having N-methyl piperazine react with following compound,
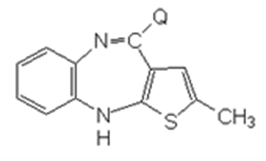
In the equation, Q is a group that may exfoliate, or
(b) having following compound set off a ring-closure reaction
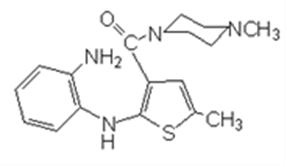
In July 2001, The Institute of Materia Medica (IMM) of the Chinese Academy of Medical Sciences (CAMS) (hereinafter referred to as Institute of Medicine), along with Watson, applied for a new drug certificate for olanzapine and its tablets, to the State Food and Drug Administration (Hereinafter referred to as SFDA). On May 9th, 2003, the Institute of Medicine and Watson obtained the New Drug Certificate for olanzapine and its tablets issued by SFDA, and Watson obtained the Drug Registration Approval for olanzapine and olanzapine tablets. The preparation method was recorded in the new drug application document “Research Materials and Literature on the Producing Methodology of API”, that is: “add 4-amino -2-methyl -10-benzyl-thieno-benzodiazepines, hydrochloride, methyl piperazine and dimethyl formamide, stir to get the crude product, with a yield coefficient of 94.5%; then add 2-methyl -10-benzyl-(4- methyl -1- piperazine)-4H- thieno- benzodiazepines, glacial acetic acid and hydrochloric acid, stir to get the crude product, with a yield coefficient of 73.2%; then with two more refinements, the total yield coefficient becomes 39.1%”. Based on the analysis of the equation, the process is to have compound in Equation 4 react with methyl piperazine to create the compound in Equation 5, and then debenzylate the compound in Equation 5 to create the compound in Equation 1. In August 2003, Watson marketed its self-produced novel antipsychotic drug “Watson-Olanzapine” to Qingdao Seventh People’s Hospital. As recorded in the product publicity materials, the main component of olanzapine tablet is olanzapine whose chemical name is 2-methyl-10-(4-methyl-1-piperazine)-4H-thieno-benzodiazepines.
In another trial, Shanghai Science and Technology Consulting Service Center issued (2010) JZ No.19 Technical Expert Report on August 25th, 2011 upon the entrustment of Jiangsu High Court. According to the said report, the active pharmaceutical ingredient (API) olanzapine could not be obtained according to the process described in the Research Materials and Literature on the Producing Methodology of API filed by Watson. The expert conclusion is that the key reaction step as recorded in the document filed by Watson to produce the API olanzapine lacks authenticity, and the filed production methodology is not feasible.
After cross-examination, Lilly accepted the expert report and Watson did not raise any objection, either. However, Watson insisted that the Two-Step Method could produce olanzapine. The failure of the experts to replicate the production method based on the filing documents is only because some contents that involved trade secrets were not included in the filing document.
Watson did not think that it had infringed upon the patent right involved for the following reasons: Watson had been using the filed olanzapine producing technology since 2003 after it submitted the supplementary application, which was approved in 2008. The filing document had been approved by SFDA on September 8th, 2010 after its feasibility was evaluated. In the absence of any evidence provided by Lilly proving Watson's production methodology, the production methodology for olanzapine filed by Watson in 2008 should be the methodology used to ascertain infringement.
It is stated in the Application Content column of the Approval Letter on Supplementary Application for Drugs submitted to SFDA by Watson on September 8th, 2010 that: “1. change the production methodology that may affect the quality of drug; 2. revise the drug registration standard”; the Approval Conclusion column states: “After review, agree to change the production methodology and revise the quality standard. There is no other change in the production methodology other than with respect to the solvents and reagents used in the preparation method on the basis of without changing the original route of synthesis. The quality standard is attached and is valid for 24 months.”
It is stated in the Descriptions of Subsection 5.1.1 “Technology Route” of Section 5.1 “Research Materials and Literature on the Production Methodology of API” in the Supplementary and Registration Information for Olanzapine attached in the Approval Letter on Supplementary Application for Drugs (2010) that: “Based on the actual production condition of the API olanzapine, we have made partial adjustment and optimization of the olanzapine preparation methodology on the basis of without changing the original reported producing route, to further guarantee and improve the quality of olanzapine intermediates and to effectively control relevant impurities during preparation process …….As neither the technology route nor the crystal-solvent used in the last step has been changed, the structure and morphology of the compound will not change.”
At the Supreme People’s Court, the court of second instance, to ascertain the technical facts involved in this case, the expert advisor of Lilly was allowed to appear in the court in accordance with the provisions of Article 79 of Civil Procedure Law of the People’s Republic of China and Article 122 of Interpretation of the Supreme People’s Court Concerning the Application of Civil Procedure Law of the People’s Republic of China (hereinafter referred to as Interpretation of Civil Procedure Law). The witness of Watson was allowed to appear in the court in accordance with the provision of Article 117 of Interpretation of Civil Procedure Law. The staff from Jiangsu Science and Technology Consulting Service Center which has issued the (2014) SJD No. 02 of Technical Expert Report were notified to appear in the court in accordance with the provisions of Article 78 of Civil Procedure Law of the People’s Republic of China and Article 227 of Interpretation of Civil Procedure Law. Technical investigators were appointed to appear in court for the first time in accordance with the provisions of Article 2 and Article 10 of Temporary Provisions of the Supreme People's Court on Several Issues Concerning the Litigation Participation by Technical Investigator of Intellectual Property Court. The Supreme People's Court asked the relevant expert advisor, witnesses and appraiser to communicate with all parties on relevant technical issues.
The Supreme People’s Court, the court of second instance, found that: Watson had signed the Technology Transfer Contract with Institute of Medicine on October 28th, 1999, pursuant to which, Institute of Medicine transfers its independently-developed anti-schizophrenia drug olanzapine and its formulation to Watson. The Institute of Medicine was responsible for completing the pre-clinical application for approval and the clinical approval in Beijing. The acceptance criteria and methods were subject to the approval standards for new drug and the acceptance was conducted by obtaining clinical approval documents and new drug certificates. In other words, both parties agreed on the new drug certificate and approval for production.
In the (J99) YSLZ No. 82 Application Form for Clinical Research of New Drugs filled and submitted by the Institute of Medicine in October 1999, the reaction route in the “Preparation Methodology” column is described as below:
On November 9th, 1999, Beijing Municipal Health Bureau issued the New Drug Development Site Assessment Report after receiving the clinical research application for new drugs from the Institute of Medicine and recorded in the “Site Assessment Conclusion” column that: “The Institute has the conditions for development of this raw material. The original records and experimental data are basically complete, and the contents are true.”
In June 2001, the Institute of Medicine and Watson jointly submitted the Application Form for New Drug Certificate and Production [(2001) JSCZ No. 019]. After receiving the application, Jiangsu SFDA issued the New Drug Development Site Assessment Report on October 22nd, 2001 and recorded in the “Site Assessment Conclusion” conclusion that: “After site assessment, the original records of sample preparation and inspection are basically complete, the inspection equipment conditions are basically in place, the research and development unit has no API production workshop at the moment, and is now applying for the new drug certificate for this product.”
According to Watson’s application, Jiangsu Food and Drug Administration issued a letter to entrust the Pharmaceutical Safety Supervision Department of Changzhou Food and Drug Administration of Jiangsu Province to conduct an inspection and sample the products at Watson’s olanzapine production site. After inspection and sampling, Jiangsu Pharmaceutical Safety Supervision Department issued the Inspection Report on Drug Registration and Production Site (reference no. CXHB0800159) and recorded in the “Inspection Result” column that: “In accordance with the requirements of the drug registration site inspection, we have inspected the production site for the first time on July 7, 2009, and found that: The company's facilities and personnel, production and inspection facilities meet the production requirements for this variety, its raw and auxiliary materials can be traced back to the source, main raw materials are supplied according to the specified quantity, and the production process is carried out according to the reported process. On August 25th, 2009, we inspected the batch manufacturing records, inspection records, raw materials requisition and use, and inventory records for the products of batch Nos. 70309001, 70309002 and 70309003, and took samples in accordance with the sampling requirement.” It is recorded in the “Comprehensive Evaluation Conclusion” column that: “According to the comprehensive evaluation, the site inspection conclusion is: Passed”.
In Subsection 5.1.2 “Technology Route” of Section 5.1 “Research Materials and Literature Materials on the Production Technology of API” in Supplementary and Registration Information for Olanzapine attached in Approval Letter on Supplementary Application for Drugs issued to Watson by the Institute of Medicine, the reaction route is described as below:
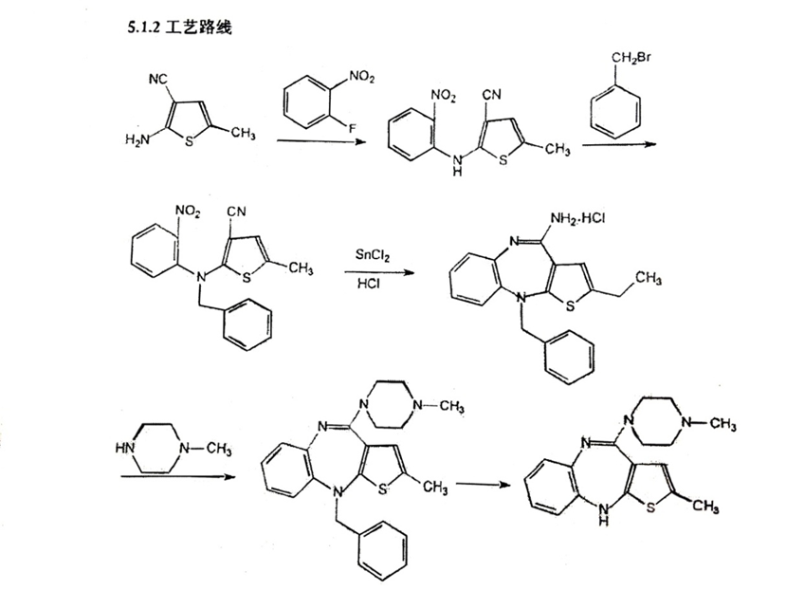
On March 5th, 2015, Jiangsu Science and Technology Consulting Center upon entrustment by Shanghai Fangda Law Firm (Beijing), issued the (2014) SJZ No. 02 Technical Expert Report, and recorded in the “Expert Conclusion” column that: “1. The olanzapine preparation methodology filed by Watson with the SFDA is feasible. 2. By comparing the olanzapine preparation methodology filed by Watson in 2008 with the SFDA, with Lilly’s methodology patent no. 91103346.7, it is found that the initial materials of both are secondary amine compounds, however, their preparation methodology differs in: 1) the key intermediates produced in the reaction are different; 2) the reaction steps are different: it is a Four-Step Method for Watson and Two-Step Method for Lilly, respectively; 3) the reaction conditions are different: the solvent used by Watson in the substitution reaction is dimethylformamide and that used by Lilly is a mixed solvent composed of dimethyl sulfoxide and methylbenzene.”
In the Court of Second Instance, Lilly clarified that it requested to protect the method (a) in Claim 1 of the patent involved in the case.
[Judgment Results]
Jiangsu High People’s Court made a civil ruling (2013) SMCZ No.0002 on October 14th, 2014 requesting: 1. Watson Pharmaceuticals (Changzhou) Co., Ltd. compensate Lilly RMB3,500,000 for its economic loss and other reasonable fees involved to deter the infringement; 2. to dismiss other claims of Lilly. With respect to the court fee of RMB809,744, Lilly should pay RMB161,950 and Watson should pay RMB647,794. Both Lilly and Watson refused to accept the ruling and appealed. The Supreme People’s Court made a civil ruling (2015) MSZZ No.1: 1. to reverse the Civil Ruling (2013) SMCZ No.0002 of Jiangsu High People’s Court; 2. to dismiss the claim of Lilly. The court fees in the First and Second Instances are RMB809,744 respectively, of which Lilly should pay RMB323,897 and Watson should pay RMB1,295,591.
[Judicial Opinions]
In the Second Instance, the Supreme People’s Court held that: it is stipulated in Article 7 of the Interpretation of the Supreme People's Court on Several Issues Concerning the Application of Law in the Trial of Cases of Infringement upon Intellectual Property Rights that: “When the People’s Court determines whether the alleged infringing technical solution falls within the scope of patent right protection, all technical features recorded in the claim by the patentee should be reviewed. In case the alleged infringing technical solution contains the same or equivalent technical features as those of the claim, the People’s Court shall determine that it falls within the scope of patent right protection; in case the alleged infringing technical solution lacks one or more technical features compared to all of those recorded by the claim, or contains one or more technical features that is (are) not the same or not equivalent with those of the claim, the People’s Court shall determine that it doesn’t fall within the scope of patent right protection.” In this case, the alleged drug produced and sold by Watson is the same as the product prepared by using the patent method involved in this case, both are olanzapine; therefore, the following three questions would be involved in order to determine whether the olanzapine preparation methodology falls within the scope of protection for the patent right involved in this case:
I. Scope of protection for patent right involved
It is stipulated in Paragraph 1, Article 56 of the Patent Law of People’s Republic of China that: “The scope of protection for the patent right to invention or utility model shall be subject to the content of its claim, both instructions and drawings can be used to interpret the claim.” In this case, Lilly required protection of the method (a) of Claim 1 of the patent right involved. The claim adopts an open composition method, in which only the tricyclic reduction, N-methyl piperazine and group participating in the substitution reaction are defined. The scope of protection covers all preparation methods to produce olanzapine by using the aforesaid tricyclic reduction and N-methyl piperazine having substitution reaction with Q group, regardless of the reaction starting materials, solvent and reaction conditions used. Otherwise, the scope of protection for the patent right involved will be reduced improperly, and the legitimate rights and interests of Lilly will be damaged.
II. Olanzapine preparation methodology actually used by Watson
It is stipulated in Paragraph 2, Article 57 of the Patent Law of People’s Republic of China that: “In case the invention patent of a new product’s manufacturing method is involved in the patent infringement dispute, the unit or individual who manufactures the same product shall provide evidence proving their manufacturing methods differ from the patented method.” In this case, neither party has any objection to the fact that the new product claimed in the patent method is olanzapine; Watson should bear the burden of proof evidencing its olanzapine preparation methodology is different from the patent method involved. Specifically, Watson should provide evidence to prove the reaction route of the olanzapine preparation methodology actually used by it does not fall within the scope of protection of the patent right involved, or else it will be liable for the legal consequences of the establishment of Lilly’s allegation for its infringement due to its inability to provide evidence.
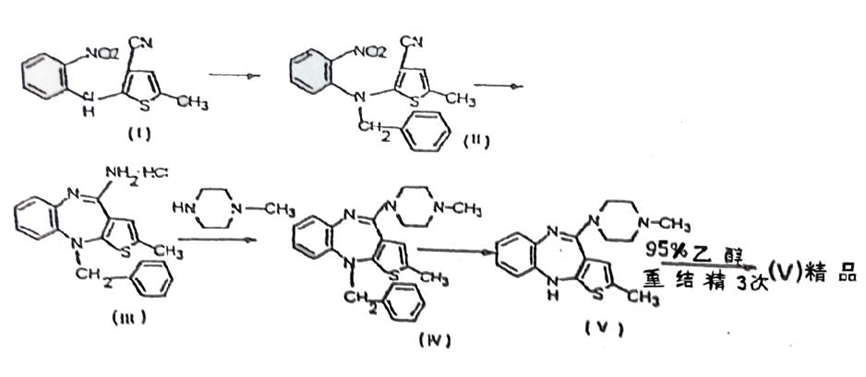
In this case, Watson claimed that it has been using a methodology that it had placed in a supplementary filing with the FSDA in 2008 since 2003, and submitted the olanzapine batch manufacturing records of 2003 and 2008 (supplementary evidence 6 in the First Instance), the production regulations of 2003, 2007 and 2013 (supplementary evidence 7 in the First Instance) and Approval Letter on Supplementary Application for Drugs (supplementary evidence 12 in the First Instance) to prove the olanzapine preparation methodology actually used by it. As mentioned above, the key to the infringement judgement of this case lies in the comparison of the reaction routes of the two technical solutions. The reaction route of the process in Watson’s 2008 supplementary filed technology can be seen in the Registration Information on the Supplementary Application for Olanzapine submitted to the SFDA, wherein Subsection 5.1.2 “Technology Route” of Section 5.1 “Research Materials and Literature Materials on the Manufacturing Technique of API” shows the reaction route as follows: First, to protect the secondary amino group of “secondary amine compound” with benzyl to produce a “benzyl compound” (benzylation) and then to have ring-closure reaction to produce “benzyl substituted thienobenzodiazepines” tricyclic compound (reduction compound). The amine group of the “reduction compound” is substituted by N-methyl piperazine to produce “condensation compound”, and lastly, the olanzapine is produced through a debenzylation reaction. The court held that there is documented evidence which can form a complete chain of evidence, proving that Watson has been using the reaction route described in 2008 supplementary filed technology since 2003 till the expiry date of the patent right involved, to produce olanzapine. The main reasons are as follows:
First, Watson submitted the supplementary application for registration of olanzapine to SFDA in the Registration Information on Supplementary Application for Olanzapine, which clearly records the reaction route of the olanzapine preparation methodology. After receiving the supplementary application, the Pharmaceutical Administration of Jiangsu Province conducted onsite inspection on the production site of Watson and made product sampling on July 7th and August 25th, 2009, respectively, and issued the Report on Production Site Inspection for Drug Registration (reference No. CXHB0800159), which shows that Watson’s “production process is conducted in accordance with the declared methodology”, three batches of products “were sampled in accordance with the sampling requirements”, and the onsite inspection conclusion is “Passed”. That means that Watson’s 2008 supplementary filed methodology is feasible, based on the onsite inspection by the Pharmaceutical Administration. Based on this, SFDA issued the Approval Letter on Supplementary Application for Drugs dated September 8, 2010 to Watson, agreeing that Watson may “change the production process and revise the quality standard” of olanzapine. The expert advisor from Lilly recognized the feasibility of Watson’s 2008 supplementary filed methodology in the Court of Second Instance. The (2014) SJZ No.02 Technical Expert Report issued by Jiangsu Science and Technology Consulting Service Center also held in the conclusion that “Watson’s 2008 supplementary filed methodology for olanzapine is feasible”. In conclusion, in absence of other evidence to the contrary, it should be presumed that Watson’s 2008 supplementary filed methodology is the olanzapine preparation methodology actually used by it after the Approval Letter on Supplementary Application for Drugs was obtained.
Secondly, Drug preparation methodology applied in large-scale industrial production is often cumbersome and complicated, due to which, its formation cannot be achieved overnight. The long-term technology accumulation process from research & development to actual production would usually involve optimization of reaction conditions and operation details based on the defects found during actual production, under the condition of maintaining basic reaction route stability. Watson’s olanzapine preparation methodology was transferred by the Institute of Medicine according to the Technology Transfer Contract dated October 28th, 1999 between the Parties, pursuant to which, the Institute of Medicine was responsible for completing the pre-clinical application for approval and the clinical approval in Beijing. In the (J99) YSLZ No.82 Application Form for Clinical Research of New Drugs filed and submitted by the Institute of Medicine in October 1999, the reaction route described in the “Preparation Methodology” column shows that the same reaction route has been used with that of Watson’s 2008 supplementary filed technology. On November 9th, 1999, the Beijing Municipal Health Bureau issued the New Drug Development Site Assessment Report based on the said clinical research application for new drug, confirming that: “The original records and experimental data are basically complete and the contents thereof are true.” Based on this, the Institute of Medicine and Watson jointly submitted the Application Form for New Drug Certificate and Production [(2001) JSCZ No.019] pursuant to the Technology Transfer Contract. After the application, Jiangsu Food and Drug Administration issued the New Drug Development Site Assessment Report on October 22nd, 2001, confirming that: “The original records of sample preparation and inspection are basically complete”. After passing all reviews including the above said assessment, the Institute of Medicine and Watson obtained the New Drug Certificate for olanzapine and olanzapine tablets, issued by the SFDA. Thus, it can be seen that Watson has used the same olanzapine preparation methodology with the reaction route stipulated in the 2008 supplementary filed methodology and had already obtained the New Drug Certificate by registering for new drug application. Therefore, it is unlikely for Watson to produce olanzapine with a very different preparation methodology before the 2008 supplementary filed technology.
Finally, it is recorded in the “Approval Conclusion” column of Approval Letter on Supplementary Application for Drugs that: “The revised production methodology has no other adjustment except with respect to the solvents and reagents used in the preparation methodology on the basis of not changing the original synthetic route”. That is to say, SFDA confirmed that the reaction route of Watson’s 2008 supplementary filed methodology is the same as that of former preparation methodology. Watson submitted the production regulations of year 2003, 2007 and 2013 as well as the olanzapine batch manufacturing records of year 2003 and 2008 in the First Instance. Since Watson claimed that the above evidence involved its trade secrets, the Court of First Instance organized both parties to make closed cross-examination in order to determine the authenticity and relevance. After the review, the court found that the olanzapine batch manufacturing records of year 2003 and 2008 are the records of actual production conducted according to the production regulations of year 2003 and 2007. Both the above production regulation and batch manufacturing records show that the basic reaction route of Watson’s olanzapine preparation methodology is the same as that it had filed in its 2008 supplementary filing. There are just some adjustments and optimization with respect to details such as reaction conditions and solvents on the basis without changing the basic reaction route. Such technology accumulation process is in line with the actual production approach.
To sum up, the court held that, Watson’s 2008 supplementary filed methodology is true and feasible, and it has been using the reaction route of supplementary methodology filed by the Institute of Medicine in 2008, since 2003 to the expiry date of the patent right involved to produce olanzapine.
III. Whether the alleged infringement claim by Lilly could be established
By comparing the reaction route of Watson’s olanzapine preparation methodology with the patent method involved, the differences between them were with respect to the reaction steps and key intermediates. To be more specific, the amine group of the tricyclic reduction used by Watson’s olanzapine preparation methodology is protected by benzyl, thereby the benzylation reaction certainly existed before the substitution reaction in order to produce the benzylated tricyclic reduction, and the debenzylation reaction step also certainly existed after the substitution reaction in order to produce olanzapine. On the other hand, there is no benzyl protection for the amine group of tricyclic reduction used by the patent involved, and there are no corresponding steps for benzylation and debenzylation.
It is stipulated in Paragraph 2, Article 17 of Several Provisions of the Supreme People’s Court Concerning the Application of Law in the Trial of Cases Involving Patent Disputes that: “The equivalent feature means to basically realize the same function and achieve the same effect by using the same means as the recorded technical features, and the features that can be associated by a person having ordinary skill in this field without creative labor, upon the occurrence of alleged infringing acts.” In this case, the differences in reaction routes between Watson’s olanzapine preparation methodology and the patent method involved lie on: firstly, the intermediate of tricyclic reduction protected by benzyl differs from that of tricyclic reduction without benzyl protection. There are differences in chemical reaction characteristics between them, namely, both the Q group and amine group on the tricyclic reduction intermediate without benzyl protection can react with N-methyl piperazine, while that on the tricyclic reduction intermediate protected by benzyl does not have undesired substitution reactions with N-methyl piperazine. The substitution reaction happens only at Q group. Correspondingly, there are no steps of benzylation and debenzylation. Therefore, there is a big difference between the two technical solutions in reaction intermediates and reaction steps. Secondly, the final product yield coefficient of Watson’s olanzapine preparation methodology is reduced due to the steps of benzylation and debenzylation compared to that of the patent method involved. Therefore, there is a big difference between the two technical solutions in technical effects such as yield coefficient. Lastly, although it is a common knowledge in the field of chemical synthesis to impose benzyl protection for the amine group of tricyclic reduction in order to reduce adverse reaction, such change is substantial. It will change the reaction characteristics of tricyclic reduction intermediate, and the added reaction step will reduce the yield coefficient. Moreover, the common knowledge of imposing benzyl protection only indicates that Watson’s olanzapine preparation methodology is relatively limited compared with the patent method involved and doesn't mean that the technical means used by the two are basically the same.
In summary, Watson’s olanzapine preparation methodology differs with the patent method involved in whether the intermediates of tricyclic reduction are benzylated intermediates and with respect to the added steps of benzylation and debenzylation. The corresponding technical features do not belong to the same technical means. There is a big difference in the technical effects achieved and no equivalent feature has been constituted. For this reason, Watson’s olanzapine preparation methodology does not fall within the scope of protection of the patent method involved.
In conclusion, Watson’s olanzapine preparation methodology does not fall within the scope of protection of the patent method involved. The Court of First Instance erred in its ascertainment of facts and application of law in its judgment, which should be corrected in accordance with the law.
[1] Collegial panel members: Zhou Xiang, Wu Rong, Song Shuhua
